Gene Editing and Drug Discovery
From Identifying Drug Targets to New Disease Therapeutics
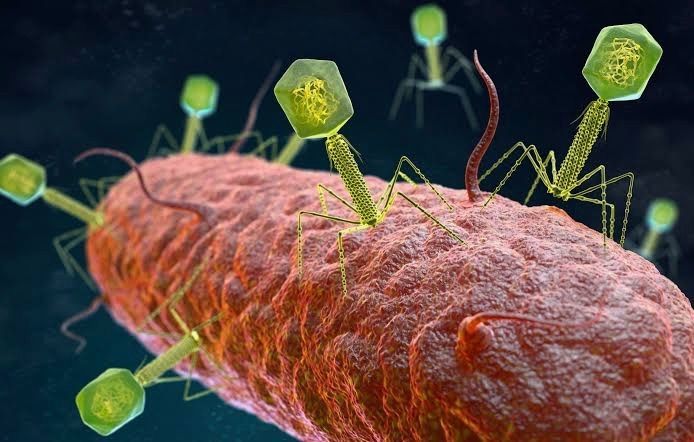
Image source: Genetic Literacy Project
Gene editing has, on occasion, been a cause of controversy , but there is no denying that this powerful technology has numerous positive uses. The impact that CRISPR-based genome engineering is having on drug discovery was covered at the inaugural ELRIG meeting on this topic held at the Kings Centre in Oxford on 27th and 28th February 2019.
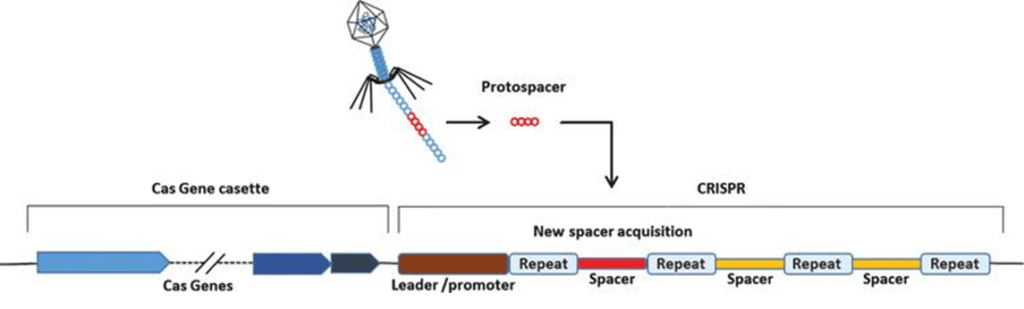
CRISPR ( C lustered R egularly I nterspaced S hort P alindromic R epeat) DNA sequences are a feature of the defence system that bacteria use to defend themselves against viruses. Snippets of the invading viral DNA are incorporated into the spacers in the CRISPR DNA sequence and act as a "photofit" reference to the DNA of the virus so that the next time the virus attacks, the bacteria remembers the virus and is able to destroy it. This is achieved by converting the viral DNA in the spacer into RNA which then attaches to the corresponding DNA piece on the attacking virus. A Cas ( C RISPR- as sociated) enzyme, produced by Cas genes located near the CRISPR sequence and attached to the RNA molecule subsequently cleaves the target DNA, rendering the virus harmless.
Image source: Lee S, et al. Journal of Cellular Biotechnology (2015)
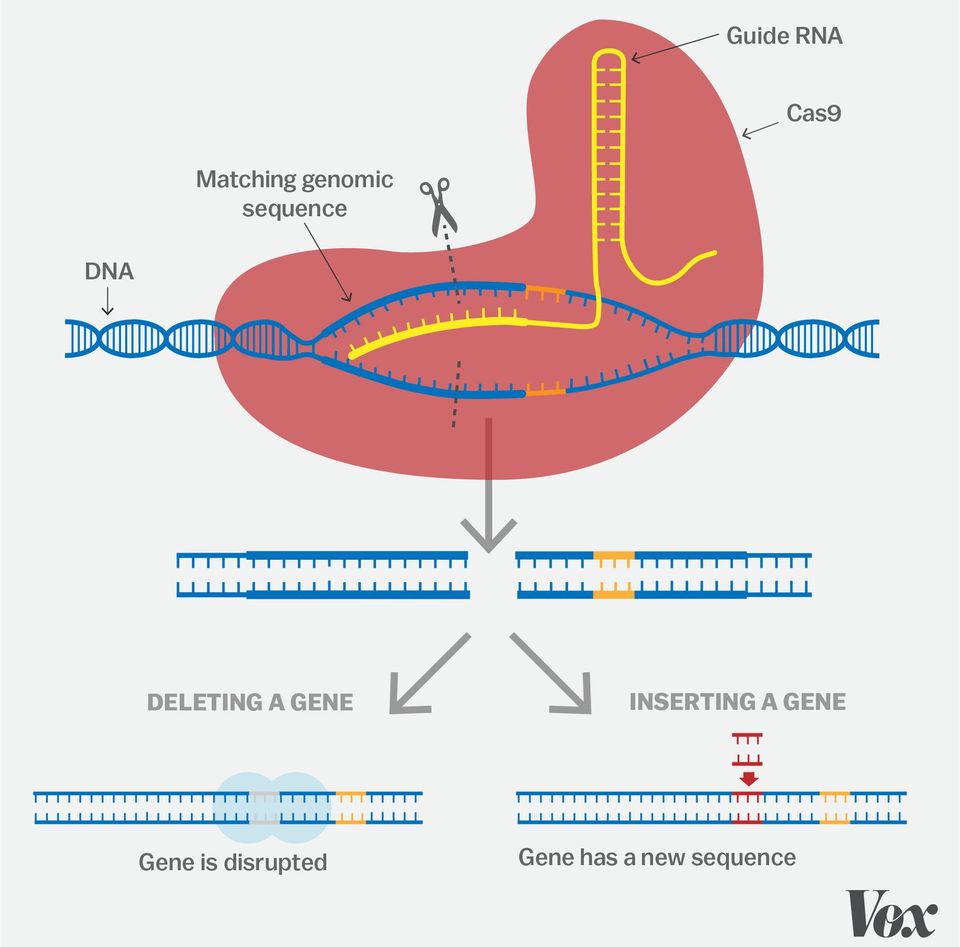
In 2012, researchers showed that the CRISPR/Cas9 system could be used to cut any genome at any specified place. Further publications in 2013 showed that the technology could be used for precise and efficient genome editing in living human and mouse cells.
Image source: Vox
Since then, the technology has literally "gone viral", with CRISPR editing being successfully used in yeast, worms, flies, fish and monkeys. This has been paralleled by interest in scientific and commercial uses of CRISPR editing, as well as ethical concerns around using the technology to create designer babies.
The 20 year backstory to the development of CRISPR
involved the work of a number of scientists in different parts of the world, so it was very appropriate that one of these pioneers, Virginijus Siksnys, from Vilnius University in Lithuania kicked off the meeting by describing his work on characterising the Cas9 nuclease, which is the variant that is most efficient at cutting human and animal DNA. This was followed by a range of talks from academic and industry scientists describing how CRISPR is impacting drug discovery from target identification and validation through to the use of CRISPR as a therapeutic modality.
By using the CRISPR/Cas9 system to activate or inhibit genes, genome wide CRISPR screens have been used to identify factors involved in cellular reprogramming (E. Metzakopian, Cambridge University), genes involved in drug resistance in BRAF mutant colon cancer (U. McDermott, AstraZeneca) and essential human genes (J. Moffatt, University of Toronto). Jon Moore (Horizon Discovery) reviewed the use of CRISPR for identifying drug targets for synthetic lethality in cancer , but stressed the importance of subsequent validation of identified hits.
In vitro cell culture models using cell lines and primary cells isolated directly from tissues continue to be a mainstay of drug discovery research. Because primary cells are isolated directly from tissues they retain normal cell morphology and many of the important markers and functions seen in vivo, at the expense of having a finite lifespan and limited expansion capacity. Although performing genome editing in low passage, primary human cells is notoriously difficult, Klio Maratou (GSK, Stevenage) described a workflow for efficient CRISPR editing in primary human lung fibroblasts. In vitro three-dimensional cell culture models in which embryonic and adult stem cells self-organise into organoids are becoming increasingly important tools in therapeutic research and development. Bon-Kyoung Koo (Institute of Molecular Biotechnology, Vienna) described how CRISPR gene editing had been used to correct a mutation in the cystic fibrosis transmembrane regulator (CFTR) locus by homologous recombination in cultured intestinal stem cells of cystic fibrosis patients and also the generation of biallelic conditional or reversible gene knockouts in various mammalian cell lines using the technique of CRISPR-FLIP.
Genetically modified animal models are a powerful way to study gene function and generate models of human disease. Ben Davies (Oxford University) described how maternal cell supply of Cas9 protein to zygotes results in increased CRISPR mutagenesis rates and how CRISPR gene editing had been used to generate mice containing a mutation in the gene GRIA3 that causes severe sleep-wake cycle dysregulation in two human brothers.
There is a lot of excitement about the use of CRISPR gene editing as a way to treat human disease and genetic defects and two talks reviewed progress in this area. Toni Cathomen (University of Freiburg) discussed using genome editing as an approach to treat diseases of the haematopoietic system and Matt Porteus (Stanford University) reviewed work on editing of hematopoietic stem cells , with reference to sickle cell disease where gene correction approaches are getting closer and closer to phase I/II clinical trials. Matt Porteus also talked about editing hematopoietic stem cells to phenotypically correct mucopolysaccharidosis type I. Successful translation of CRISPR-based therapeutics into the clinic will depend on identifying and minimising deleterious off-target mutations introduced during the gene editing process and Marcello Maresca (AstraZeneca, Cambridge) described an experimental strategy for defining and quantifying gene-editing nuclease off-target effects in whole organisms.
To complement the talks described above, a number of companies gave presentations on services, reagents and equipment that can be used to aid the CRISPR gene editing workflow:
| Company |
Product |
| Arrayed CRISPR screening libraries |
|
| CRISPR screening services |
|
| CRISPR reagents |
|
| Custom gene synthesis and novel mutant Cas proteins |
|
| Liquid handling for CRISPR workflow |
|
| Human iPS-derived disease model lines for drug screening |
|
| Automated liquid handling equipment |
|
| Automated liquid handling equipment |
|
| Automated pipetting devices |
|
| High throughput single cell cloning of CRISPR-edited cell lines |
|
| GMP manufacturing of Cas9 protein for clinical studies |
In addition to the talks there was an accompanying scientific poster session with over 20 posters from various companies and academic groups covering a range of areas from optimising CRISPR vector systems through to microfluidic systems for automated gene editing. Two of the posters were presented in short poster spotlight talks given by Anne Goeppert (AstraZeneca, Cambridge) and Barbara Mair (University of Toronto) on using CRISPR for the generation of disease models in immune cells and the use of CRISPR-on-a-chip to screen for modifiers of CD47 expression, respectively.
All in all, a well organised and attended informative meeting on the use of CRISPR for drug discovery and the first in an increasingly popular series of free to attend meetings organised by ELRIG in 2019.