Image Source Oseltamivir (Tamiflu) is an antiviral medication used to treat influenza (flu) . It works by blocking neuraminidase on the surface of influenza viruses, preventing the virus from leaving infected cells and spreading to new cells, thereby inhibiting the influenza virus's ability to spread within the body. Oseltamivir is most effective when started within 48 hours of symptom onset. It can reduce the duration and severity of influenza symptoms, and it may also reduce the risk of lower respiratory tract complications. Current WHO guidelines recommend against the use of oseltamivir for patients with non-severe (uncomplicated) influenza , but conditionally recommend the use of oseltamivir for patients with severe (complicated) influenza , including infection with novel influenza A viruses associated with high mortality, or unknown risk of severe disease . In the UK, NICE and UKHSA guidelines do not indicate use of oseltamivir in people who were previously healthy, unless the person is at significant risk of developing serious complications from influenza. Older adults, pregnant women, people who are immunosuppressed and those with certain chronic health conditions fall into this latter category. In the US oseltamivir treatment is recommended for all patients hospitalized with severe influenza regardless of illness duration, with its use in uncomplicated influenza left to clinical judgement . In the case of severe influenza, a 2021 observational study reported that early (within 48 h) oseltamivir treatment was associated with improved survival rates in critically ill patients with influenza pneumonia , and may decrease ICU length of stay and mechanical ventilation duration. However, a 2024 systematic review and network meta-analysis of 73 trials involving 34,332 participants, concluded that oseltamivir had little or no effect on mortality and admission to hospital, likely had no important effect on time to alleviation of symptoms, and likely increased adverse events related to treatments. Although the validity of the analysis has been questioned , the findings have informed WHO guidelines. A recent 2025 study retrospective cohort study using target trial emulation of 11 073 patients hospitalized for severe influenza found that patients treated with oseltamivir were less likely to die in hospital, more likely to be discharged alive earlier, less likely to be transferred to the ICU, and less likely to be readmitted to hospital after discharge. The absolute risk reduction for mortality was −1.8%, so the effect was small but clinically significant in terms of benefit in severe influenza. Whilst clinical guidelines recommend the use of oseltamivir in patients hospitalised with severe influenza, the studies above indicate that the effects on mortality are modest. As a result, there is still a need for therapeutic interventions that can decrease mortality and reduce the duration of hospitalisation. The p38MAPK inhibitor POLB001 has great potential to do this, particularly as it does not affect the antiviral activity of oseltamivir, so it can be given to patients concurrently.
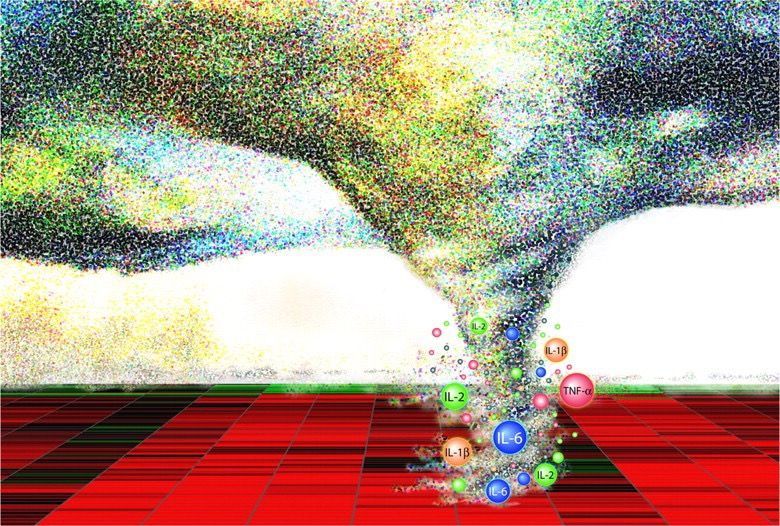
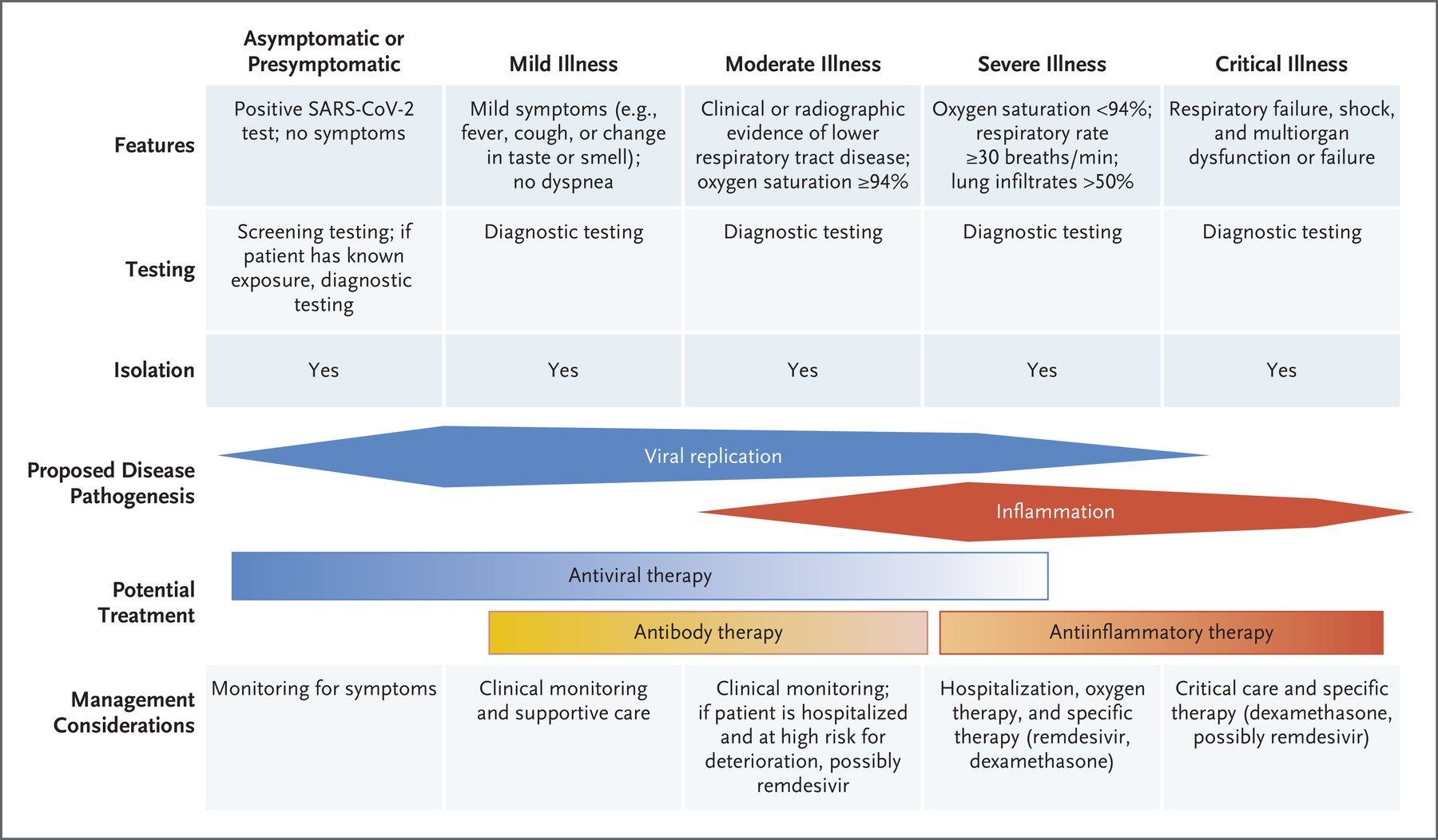
Image Source COPD exacerbations are periods where patients with COPD experience worsened symptoms , characterised by sudden increases in breathlessness and cough, and changes in sputum production (amount, colour, and/or thickness). Respiratory infections (caused by viruses or bacteria), environmental pollutants, smoking, or even changes in weather, can trigger exacerbations in COPD patients. Smoking cessation, vaccination, and management of other health conditions can help reduce the frequency and severity of exacerbations. Recognizing the early signs of exacerbations, so that medical attention can be sought promptly, is key to the effective management of exacerbations and preventing complications. Treatment of exacerbations can range from the use of bronchodilators, corticosteroids and antibiotics, to oxygen therapy, and even mechanical ventilation in hospital. Although type 1, neutrophilic inflammation of the lungs is a prominent feature of COPD, with increased neutrophil levels correlating with lung function decline and disease progression , between 20% and 40% of COPD patients exhibit a prominent type 2, eosinophilic inflammation in their lungs, which is considered a distinct phenotype within COPD that increases exacerbation risk. As a result, biologics which target type 2 inflammation are now emerging as a new class of therapies for COPD exacerbations. Dupilumab , a fully human monoclonal antibody, which blocks the shared receptor component for interleukin-4 and interleukin-13 (key drivers of type 2 inflammation), has recently been approved by the US Food and Drug Administration(FDA) for use in treating patients with uncontrolled COPD and an eosinophilic phenotype. In clinical trials, COPD patients with type 2 inflammation (as indicated by elevated blood eosinophil counts), who received dupilumab had fewer exacerbations, better lung function and quality of life, and less severe respiratory symptoms than those who received placebo. More recently, a phase 3 randomised trial found that the humanised monoclonal antibody mepolizumab reduced COPD exacerbations by targeting the cytokine interleukin 5 (IL-5) , which plays a central role in eosinophilic inflammation. Patients with COPD, a history of exacerbations, and a high eosinophil blood count received monthly injections of either mepolizumab or a placebo, in addition to continued background treatment with triple inhaled therapy. Treatment with mepolizumab led to a lower annualized rate of moderate, or severe exacerbations, when added to background triple inhaled therapy . The FDA has now approved mepolizumab as the first once-monthly biologic for COPD with eosinophilic phenotype. COPD is a complex disease with various inflammatory pathways , so the use of biologic therapies to target specific inflammatory pathways in COPD patients is a significant advancement in disease treatment . Biologics offer a new way to target specific types of inflammation, potentially improving lung function and reducing exacerbations in certain COPD patients, and are typically used as an add-on treatment to existing therapies like inhaled corticosteroids, long-acting bronchodilators, and other medications . The therapeutic goals of biologics remain the same as with other treatments for COPD: restoration of normal inflammatory response; and alteration of disease progression. The best biologic for a specific patient will depend on their individual characteristics and the type of inflammation driving their COPD. Ongoing research is exploring the potential of various biologics for COPD , including those targeting other inflammatory pathways.
Modified from Image Source Animal studies in sheep and mice , as well as evidence from trials in humans and case studies of compassionate clinical use, indicate that phage therapy is efficacious, safe , and non-toxic . A 2022 systematic review of clinical data obtained from phage therapy clinical trials, safety trials, and case studies between 2000 and 2021 for difficult to treat bacterial infections in several medical disciplines, concluded that phage therapy given via different routes of administration is well tolerated and safe with a low incidence of side effects. Unfortunately, heterogeneity between different clinical studies precluded a meta-analysis of the data, highlighting the need for high quality clinical trials to improve knowledge on long-term patient and disease outcomes. The use of purified phage to treat superficial bacterial infections appears to be efficacious safe, and side effect free, even when delivered by invasive routes of administration (e.g. intravenous and intra-articular ), or used in immunocompromised patients. Some would argue that phage therapy appears to have a better safety profile than antibiotics , and has a minimal impact on commensal flora , thus reducing the likelihood of opportunistic infections. Consistent with our natural exposure to phages, there are no reports of allergic responses to phages, potentially making them suitable alternatives for patients with antibiotic hypersensitivity. Because phages can kill bacterial cells quickly , release of endotoxins from lysed bacterial cells in severe infections is a potential safety concern. However, it has been demonstrated that phage lysis releases less endotoxin than beta-lactam antibiotics. Another potential issue with phage therapy is that phage cocktails might have effects on non-targeted bacteria and so affect the human microbiome. However, phage therapy does not appear to have an adverse effect on gut microbiota and has less of an effect on gut diversity than antibiotics, having a beneficial effect on gut health. Studies have suggested that bacteriophages can interact with eukaryotic cells, significantly influencing the functions of tissues, organs, and systems of mammals, including humans. In addition, there are concerns regarding the long-term impact of phages on the human immune system. In terms of effects on innate immunity , phages appear to induce anti-inflammatory responses. In terms of effects on the adaptive immune response , phages can be immunogenic, but are not very effective at inducing a specific immune response. With regards to phages inducing anaphylaxis , no cases have been reported. Autoimmune responses to phage therapy are a possibility, however, their immunomodulatory role, particularly in curbing inflammation means that phage therapy is being explored for the treatment of autoimmune liver diseases . Although the indications are that phage therapy is safe, with few adverse effects, current research on phage safety monitoring lacks sufficient and consistent data for regulatory purposes, which would require a standardized phage safety assessment to ensure a robust evaluation of the safety profile of phage therapy. Although the Australian STAMP protocol is not a randomised clinical trial, it does provide a framework for the collection of higher quality efficacy and safety data than individual case studies.
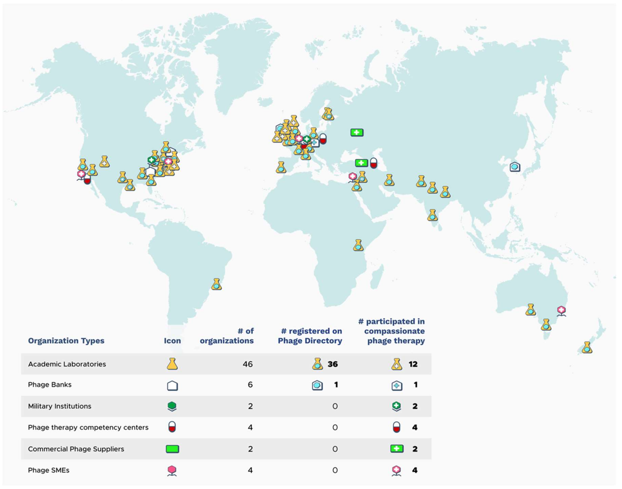
Image Source In this second article about phage therapy, I will be focussing on its use in different countries, with special emphasis on the UK. Phage Therapy in the UK In the UK, phages are classed as a biological medicine and none are licensed for clinical use. As a result, phage therapy is applied on a compassionate use basis as an unlicensed medicinal product (a “ special ”). Phages imported for use as an unlicensed medicine in the UK do not need to be manufactured according to Good Manufacturing Practice ( GMP ), however, the Medicines and Healthcare products Regulatory Agency ( MHRA ) must be notified at least 28 days prior to importation, and doses imported are limited to a small number. Paradoxically, phages manufactured in the UK must be produced to GMP, including phage for use in clinical trials. As a result, the current clinical provision of phage therapy in the UK is ad hoc and relies heavily upon networking with international sources of phages , including organisations such as Phage Directory , who help connect clinicians who want phages for clinical use, with groups who have appropriate phages. The MHRA recently published a document on the regulatory considerations for therapeutic use of bacteriophages in the UK. According to Phage-UK there have been 24 clinical trials involving phage therapy since 2020. Phage Therapy in Other Countries Globally, different countries have different regulatory frameworks for the clinical use of phage therapy. Eastern European countries have over 100 years of phage therapy experience. Russia and Ukraine allow open use and commercialisation of phage products. Phages are a standard medical application in Georgia . In Poland , specialised institutes have supplied personalised phage products to physicians since 2005. In the European Union (EU) , phage therapy is not approved as a standard medicinal product for human use. Like the UK, It is primarily used in compassionate use cases, clinical trials, or for individual experimental therapy attempts. Although a European Medicines Agency (EMA) guideline exists for veterinary bacteriophage medicinal products , there is currently no corresponding regulatory guidance for human use of such products in the EU. The EMA opened a public consultation on a concept paper on the development and manufacture guidelines for human bacteriophage medicinal products tailored to phage therapy on 23 rd December 2023, which ended on 31 st March 2024. In the meantime, a regulatory roadmap for phage therapy under EU pharmaceutical legislation has been published . Belgium has implemented a phage therapy framework focusing on magistral phage preparations that allows patients to actively seek access to personalized phage therapy. In the magistral approach , individual phages are prepared according to a phage monograph (a standardized document that provides detailed information about a specific bacteriophage, its properties, and its potential use in phage therapy), and reference laboratories provide quality control (QC) testing. Clinicians prescribe phage cocktail preparations for use in specific patients, which are then prepared by pharmacists. Based on the Belgian model, a general chapter on phage therapy medicinal products was published in 2024 by the European Pharmacopeia . In Australia , all clinicians and researchers within the Phage Australia network have adopted the Standardised Treatment and Monitoring Protocol for Adults and Paediatric Patients ( STAMP ). STAMP is a clinical protocol for administering and monitoring phage therapy, rather than the phage product. As a result, STAMP looks at the process, not the product and means that different patients can be treated with different phages at different sites of infection, but the treatment protocol is standardised. The STAMP protocol has been approved by Australia's national ethics committee and endorsed by Australia's national infectious disease physician society, as well as its paediatric arm. In the US , phage therapy is not yet an FDA licensed treatment. However, it available under a special programme called the “ expanded access eIND system ” . For patients who have exhausted standard-of-care therapy, an application is submitted to the FDA by the treating physician, where a patient meets a list of criteria. UC San Diego's IPATH is the first dedicated phage therapy centre in North America. They focus on treating patients with life-threatening multi-drug resistant infections through the FDA's compassionate use program. IPATH also works to advance phage therapy into clinical trials and provides guidance to physicians worldwide on phage therapy protocols. The PhagesDB database details over 25,000 new phages identified by the SEA-PHAGES programme at the University of Pittsburgh. One particular phage from this collection, called Muddy, has been used therapeutically in a cystic fibrosis patient infected with a multi-drug resistant strain of Mycobacterium abscessus. Creative Biolabs is developing libraries of characterised phages, as sell as platforms for identifying and then producing phages to GMP standard for formulation and delivery. Israel focusses on compassionate use of phage therapy, with the Israeli Phage Therapy Center conducting all of the steps required, from phage isolation and characterization to treatments for non-resolving bacterial infections. In India , phage therapy is offered as compassionate Phage Therapy regulated by the Declaration of Helsinki and coordinated by the Central Drugs Standard Control Organization. Vitalis Phage Therapy has created a framework for patients to access phage therapy in India. In China , there are two routes to using phage therapy applications. Phage products with fixed ingredients are regulated as innovative biological products . Personalized phage therapies, on the other hand, need to go through investigator-initiated trials (IIT) and, if successful, the phage therapeutic can then be used at certain institutions. Expanding the Use of Phage Therapy in the UK To progress past the ad hoc use of phage therapy in the UK, the infrastructure to support the route from patient enrolment, through isolation and identification of pathogenic bacteria and therapeutic phages, to formulation, administration and monitoring of efficacy, as well as phage resistance, will need to be put in place. A key requirement for clinicians in the UK will be the ability to access phages that are efficient at killing the strain of bacteria causing an infection. A roadmap for the delivery of clinical phage therapy to the UK has been proposed, which would require: expansion of existing phage biobanks; the development of both personalised treatments for individual patients; and off-the-shelf phage cocktails that could be used to treat large numbers of patients. Innovate UK has developed the Phage Innovation Network to help drive the use of phage based therapy in the UK and build on the expertise of the many phage experts based in the UK. To enable this, systematic libraries of a diverse array of phages that are well characterised and curated, as well as manufactured to GMP standard will be needed. The Citizen Phage group at Exeter University , uses volunteer citizen scientists to collect samples from a wide range of environments to facilitate the laboratory identification of new phages for therapeutic use. The UK Phage Library at the University of Leicester is aiming to develop libraries of phages which can be screened against specific bacterial strains to identify phages they are sensitive to. Unfortunately, these phages cannot be used in patients in the UK due to lack of GMP phage manufacturing capability, but they are provided for use in other countries whose regulatory frameworks permit their use. On the commercial side, Nexabiome in Glasgow aims to provide an end-to-end service covering phage identification and isolation, to production and formulation. Establishing a UK Phage Manufacturing Facility that can produce phage preparations for both commercial and non-commercial customers that are suitable for administering to patients would be a key requirement for widening the use of phage therapy in the UK. Towards this end, UK Phage Therapy is working with public and private partners to establish a centralised phage susceptibility testing and GMP phage production facility in the UK via the Centre for Process Innovation (CPI) . The Centre for Phage Research is also working with regulators, policymakers and other stakeholders to establish frameworks and pathways to enable public access to phage products. Whether the UK will remove the requirement for GMP manufacture of phages for Phase I clinical trials, as is the case in the US, is unclear. However, phase II/III trials would still require GMP manufacture for off-the-shelf phage products. Phage-UK has developed a standardised protocol for the treatment and monitoring of phage therapy in UK patients suffering from cystic fibrosis and bronchiectasis , where there is no alternative treatment available. The protocol, based upon the Australian STAMP protocol, is a document that assists clinicians making a submission to their NHS Hospital Board for approval to use phage therapy on a named patient basis. Presumably, this protocol could be adapted for the treatment of UK patients with other serious infections such as urinary tract infections (UTIs), prosthetic joint infections (PJIs) and sepsis. A benefit of using such a standardised protocol would be the collection of safety and efficacy data on phage therapy that could then inform the design of subsequent clinical trials of phage therapy in the UK. Conclusion For most countries, compassionate use is the major pathway for patients to access phage therapy. Belgium (with the magistral approach), and Australia (with STAMP), currently lead the way in developing frameworks to facilitate patient access to phage therapy. The lack of such frameworks in other countries, including the UK, reflect the view that phage therapy is still an experimental treatment that requires more convincing clinical evidence of efficacy. In the UK, it will be interesting to see if any of the recommendations made in the March 2024 Policy Paper on the antimicrobial potential of bacteriophages, published under the Conservative government in power at the time, are followed up by the current Labour government.
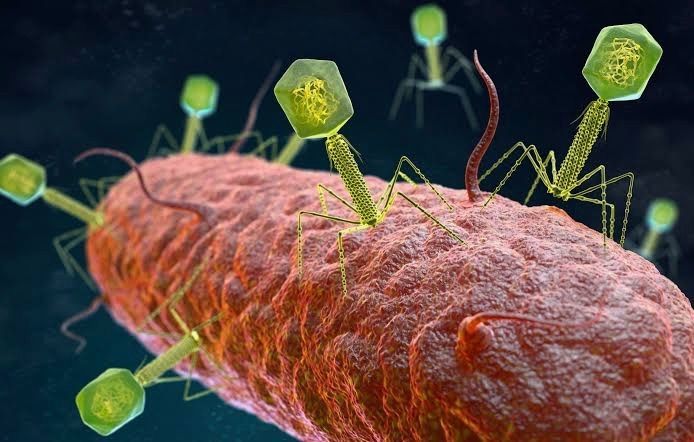
Image Source: The growing global problem of antibiotic resistance , and the lack of new antibiotics being developed, has rekindled an interest in phage therapy: the use of viruses (bacteriophages) that specifically target and kill bacteria, as a treatment for antibiotic resistant infections in humans. This article is an overview of phage therapy and is the first in a series where I will explore aspects of this technology in greater detail. Links to papers and websites that contain diagrams or graphics relevant to this article are provided at the end of this article, as are links to PubMed search results for phage therapy papers, reviews and clinical trials. Bacteriophages In 1986, nanotechnology pioneer K. Eric Drexler imagined a dystopia where invisible self-replicating nanobots proliferated voraciously and took over the entire planet. Spooked by Drexler’s nightmare, Prince Charles (the heir to the British throne at the time, but now King Charles III) requested that the eminent Royal Society investigate the risks that nanotechnology posed. However, the reality is that nano-scale self-replicating voracious killers have existed on earth for over 4 billion years. They can make hundreds of copies of themselves in as little as 15 minutes, and are found in vast numbers everywhere on our planet. Thankfully, they are not harmful to humans. Instead, they infect and destroy bacteria in a process perfected over the eons. Known as bacteriophages , these biological entities were discovered at the beginning of the twentieth century. Bacteriophages (usually referred to as phages - derived from the Greek word “phagein”, meaning “to devour/eat”) are viruses composed of a nucleic acid genome encased in a phage-encoded protein capsid shell. Phages are found in three basic structural forms : icosahedral head with a tail; icosahedral head without a tail; and filamentous. They infect and kill bacteria by replicating inside bacterial cells, then breaking open ( lysing ) the infected cells before releasing large numbers of new progeny phage particles. In the laboratory, this process is visualised and monitored using plaque assays . Phages are ubiquitous and diverse . It has been estimated that there are 10 31 bacteriophages on the planet, more than every other organism on Earth, including bacteria. Phages can be isolated from sources where high numbers of bacteria occur, such as human sewage , soil , rivers , faeces , even slime in a stream . Phages are specific to individual bacterial species and strains and do not infect mammalian cells. However, because of the microbiome , the human body contains large numbers of phage particles and varieties of phages (the so called phageome ) and, as a result, phage can interact with mammalian cells. Phages are fascinating biological entities. As an undergraduate biology student I learned of the importance of phages as key experimental tools in the development of the fields of molecular genetics and molecular biology. As a post-graduate biochemistry student I worked with phages in the lab of the late Pauline Meadow , who used them as a way of identifying lipopolysaccharide (LPS)-defective mutants of Pseudomonas aeruginosa. As a doctoral student in molecular biology working on DNA methylation in the slime mould Physarum polycephalum, and as a postdoctoral researcher working on human genome analysis, I used phage lambda cloning vectors to construct genomic DNA libraries for gene isolation (e.g. tubulin genes from the parasite Trypanosoma brucei) and for the physical mapping of human genomic DNA (e.g. the human dystrophin-encoding gene using a specially modified phage lambda vector I developed). The Challenge of Bacterial Anti-Microbial Resistance More widely referred to as anti-microbial resistance , or AMR , bacterial AMR (bAMR) occurs when bacteria develop the ability to defeat the antibiotics designed to kill them. This resistance can result from several different mechanisms . I n this article I am referring specifically to bAMR, and not viral, fungal, or parasitic AMR . A systematic analysis published in 2022 estimated that there were 4.95 million deaths associated with bAMR globally in 2019. As a result, bAMR has the potential to affect each and every one of us by impacting the treatment of illnesses, surgical procedures and cancer treatment, as well as increasing rates of death. The increase in prevalence of bAMR has led to fears of future pandemics caused by drug-resistant bacteria. In the UK, the Government and the National Health Service (NHS) have both developed action plans to tackle bAMR which emphasise optimising and reducing exposure to antibiotics. Despite these measures, however, new antibiotics and alternatives to antibiotics are still needed, particularly in cases where infections are refractory to antibiotic use. Developing new classes of antibiotics is challenging . The greater portion of recently approved antibiotics have tended to be derivatives of existing classes of drug compounds. Although pharmaceutical giant Roche recently reported the identification of a promising new class of antibiotic molecules that target carbapenem-resistant Acinetobacter baumannii (CRAB), many big pharma companies have ceased antibiotic development. As a result, small biotech companies are leading research and development efforts in this area. Phage Therapy Case studies in the scientific literature , and success stories described in the press and in books have highlighted the use of phage therapy to treat infections caused by antibiotic-resistant bacterial strains. However, phage therapy as a way of treating bacterial infections is not new. Phages were first used to treat bacterial infections in 1919 (the “pre-antibiotic era”), but the approach never really gained traction in the West, particularly after penicillin was discovered in 1928 and became the favoured way to treat bacterial infections in the 1940s. Despite this, phages have continued to be used in Russia , Georgia and Poland as an alternative to antibiotics since the early 20 th century . The excellent book “The Good Virus” by Tom Ireland, gives a vivid and detailed account of the history of phage therapy and how interest in it as an approach to treating bacterial infections has waxed and waned over the past century. Now, because of the growing threat of bAMR, there has been a resurgence of interest in using phages to tackle antibiotic resistant infections. As some phages degrade biofilms, phage therapy also potentially provides a way to deal with the antibiotic tolerance seen in some chronic diseases resulting from biofilm production (e.g. cystic fibrosis ). Also of interest is the potential to use phage resistance as a way to steer bacteria towards an antibiotic-sensitive phenotype. Unfortunately, in many parts of the world current regulations restrict the application of phage therapy to individual 'compassionate use ' in patients with infections where antibiotics have failed. In the UK phage therapy has been used sparingly to treat Pseudomonas and Mycobacterial infections mainly due to the lack of sustainable access to phages manufactured to good manufacturing practice ( GMP ) standard. The first successful clinical trial of phage therapy in the UK was published in 2009. Since then, phage therapy has been used in the treatment of patients with diabetic foot ulcers and cystic fibrosis . There has often been a difference between the results of individual real world case reports of successful phage therapy and the results of larger scale studies. Therefore, high quality clinical trials of phage therapy in the treatment of a range of conditions are needed to provide a solid evidence base on the efficacy and safety of phage therapy in human patients to support wider clinical use. A retrospective observational analysis of 100 consecutive cases of personalised phage therapy carried out by a Belgian consortium using combinations of 26 bacteriophages and 6 defined bacteriophage cocktails reported clinical improvement and eradication of targeted bacteria for 77.2% and 61.3% of infections, respectively. However, eradication was 70% less when antibiotics were not used concurrently. Recently, there have been calls for the British Government to invest in phage therapy as a way to tackle bAMR. As a first step, a report published in January 2024, following a Parliamentary Inquiry in 2023, recommended that the British Government bring together phage experts and stakeholders (scientific, clinical and regulatory) to assess what would be required to enable phage therapy to be used more widely in the National Health Service (NHS) and other UK healthcare settings. A Government response to this report which supports these recommendations and makes 18 additional recommendations across 4 themes, has now been published . The Innovate UK Phage Knowledge Transfer Network has been established to provide a forum for funders and phage researchers to discuss these matters and ways forward, including multi-party collaborations and co-investments by public and/or private funders. Discussion The dramatic results seen in sick people who have received phage therapy as a last ditch treatment when conventional antibiotic therapy has failed, provides a compelling narrative for its potential in the treatment of bAMR. However, the body of evidence required to convince regulatory authorities, governments and the medical establishment of phage therapy efficacy is clearly lacking at the moment. Even if that data is forthcoming, it is unlikely that phage therapy will ever replace antibiotics. More likely, phage therapy will continue to be used in a personalised way to treat infections that are resistant to standard antibiotic therapy, but in the future it is reasonable to envisage clinical scenarios where phages might be used in conjunction with antibiotics. The judicious use of phages might help protect and preserve existing antibiotics and combining the two appears to be more effective than either on their own. Phage therapy may also be useful for treating people who are allergic to antibiotics and may not have other treatment options. It is, of course, entirely possible that phage therapy for humans never progresses past the stage it is at now. Maybe new classes of antibiotics will be discovered. Maybe other antimicrobial therapeutic modalities will be invented, or discovered. Hopefully, though, the many initiatives taking place worldwide will result in the development of a safe and clinically proven version of phage therapy that will become part of an expanded therapeutic toolkit. However, what is clear at present is that while these challenges are being tackled, phages are already being deployed in various animal and non-medical scenarios such as food safety and environmental pathogen control (e.g. aquaculture ). As I explored the extensive literature around phage therapy , I realized that I could only scrape the surface of this subject in such a short article. But it is a fascinating field with therapeutic potential. Therefore, in later blog articles, I will discuss aspects of phage therapy; such as safety, phage production, commercialisation, and the regulatory approaches to using phage therapy in different countries round the world, including the UK, in more detail. Links to websites and papers with relevant diagrams and graphics: Structure of a bacteriophage Bacteriophage life cycle Phage lambda structure How bacteriophages infect and lyse bacterial cells Phage therapy Bacteriophage plaque assay Overview of antimicrobials Antibiotic Resistance Causes of antibiotic resistance Antimicrobial resistance worldwide PubMed literature links: Bacteriophage papers Bacteriophage therapy papers Bacteriophage therapy reviews Bacteriophage therapy randomised controlled trials Phage therapy systematic reviews

Image Source Oseltamivir (Tamiflu) is an antiviral medication used to treat influenza (flu) . It works by blocking neuraminidase on the surface of influenza viruses, preventing the virus from leaving infected cells and spreading to new cells, thereby inhibiting the influenza virus's ability to spread within the body. Oseltamivir is most effective when started within 48 hours of symptom onset. It can reduce the duration and severity of influenza symptoms, and it may also reduce the risk of lower respiratory tract complications. Current WHO guidelines recommend against the use of oseltamivir for patients with non-severe (uncomplicated) influenza , but conditionally recommend the use of oseltamivir for patients with severe (complicated) influenza , including infection with novel influenza A viruses associated with high mortality, or unknown risk of severe disease . In the UK, NICE and UKHSA guidelines do not indicate use of oseltamivir in people who were previously healthy, unless the person is at significant risk of developing serious complications from influenza. Older adults, pregnant women, people who are immunosuppressed and those with certain chronic health conditions fall into this latter category. In the US oseltamivir treatment is recommended for all patients hospitalized with severe influenza regardless of illness duration, with its use in uncomplicated influenza left to clinical judgement . In the case of severe influenza, a 2021 observational study reported that early (within 48 h) oseltamivir treatment was associated with improved survival rates in critically ill patients with influenza pneumonia , and may decrease ICU length of stay and mechanical ventilation duration. However, a 2024 systematic review and network meta-analysis of 73 trials involving 34,332 participants, concluded that oseltamivir had little or no effect on mortality and admission to hospital, likely had no important effect on time to alleviation of symptoms, and likely increased adverse events related to treatments. Although the validity of the analysis has been questioned , the findings have informed WHO guidelines. A recent 2025 study retrospective cohort study using target trial emulation of 11 073 patients hospitalized for severe influenza found that patients treated with oseltamivir were less likely to die in hospital, more likely to be discharged alive earlier, less likely to be transferred to the ICU, and less likely to be readmitted to hospital after discharge. The absolute risk reduction for mortality was −1.8%, so the effect was small but clinically significant in terms of benefit in severe influenza. Whilst clinical guidelines recommend the use of oseltamivir in patients hospitalised with severe influenza, the studies above indicate that the effects on mortality are modest. As a result, there is still a need for therapeutic interventions that can decrease mortality and reduce the duration of hospitalisation. The p38MAPK inhibitor POLB001 has great potential to do this, particularly as it does not affect the antiviral activity of oseltamivir, so it can be given to patients concurrently.

Image Source COPD exacerbations are periods where patients with COPD experience worsened symptoms , characterised by sudden increases in breathlessness and cough, and changes in sputum production (amount, colour, and/or thickness). Respiratory infections (caused by viruses or bacteria), environmental pollutants, smoking, or even changes in weather, can trigger exacerbations in COPD patients. Smoking cessation, vaccination, and management of other health conditions can help reduce the frequency and severity of exacerbations. Recognizing the early signs of exacerbations, so that medical attention can be sought promptly, is key to the effective management of exacerbations and preventing complications. Treatment of exacerbations can range from the use of bronchodilators, corticosteroids and antibiotics, to oxygen therapy, and even mechanical ventilation in hospital. Although type 1, neutrophilic inflammation of the lungs is a prominent feature of COPD, with increased neutrophil levels correlating with lung function decline and disease progression , between 20% and 40% of COPD patients exhibit a prominent type 2, eosinophilic inflammation in their lungs, which is considered a distinct phenotype within COPD that increases exacerbation risk. As a result, biologics which target type 2 inflammation are now emerging as a new class of therapies for COPD exacerbations. Dupilumab , a fully human monoclonal antibody, which blocks the shared receptor component for interleukin-4 and interleukin-13 (key drivers of type 2 inflammation), has recently been approved by the US Food and Drug Administration(FDA) for use in treating patients with uncontrolled COPD and an eosinophilic phenotype. In clinical trials, COPD patients with type 2 inflammation (as indicated by elevated blood eosinophil counts), who received dupilumab had fewer exacerbations, better lung function and quality of life, and less severe respiratory symptoms than those who received placebo. More recently, a phase 3 randomised trial found that the humanised monoclonal antibody mepolizumab reduced COPD exacerbations by targeting the cytokine interleukin 5 (IL-5) , which plays a central role in eosinophilic inflammation. Patients with COPD, a history of exacerbations, and a high eosinophil blood count received monthly injections of either mepolizumab or a placebo, in addition to continued background treatment with triple inhaled therapy. Treatment with mepolizumab led to a lower annualized rate of moderate, or severe exacerbations, when added to background triple inhaled therapy . The FDA has now approved mepolizumab as the first once-monthly biologic for COPD with eosinophilic phenotype. COPD is a complex disease with various inflammatory pathways , so the use of biologic therapies to target specific inflammatory pathways in COPD patients is a significant advancement in disease treatment . Biologics offer a new way to target specific types of inflammation, potentially improving lung function and reducing exacerbations in certain COPD patients, and are typically used as an add-on treatment to existing therapies like inhaled corticosteroids, long-acting bronchodilators, and other medications . The therapeutic goals of biologics remain the same as with other treatments for COPD: restoration of normal inflammatory response; and alteration of disease progression. The best biologic for a specific patient will depend on their individual characteristics and the type of inflammation driving their COPD. Ongoing research is exploring the potential of various biologics for COPD , including those targeting other inflammatory pathways.

Modified from Image Source Animal studies in sheep and mice , as well as evidence from trials in humans and case studies of compassionate clinical use, indicate that phage therapy is efficacious, safe , and non-toxic . A 2022 systematic review of clinical data obtained from phage therapy clinical trials, safety trials, and case studies between 2000 and 2021 for difficult to treat bacterial infections in several medical disciplines, concluded that phage therapy given via different routes of administration is well tolerated and safe with a low incidence of side effects. Unfortunately, heterogeneity between different clinical studies precluded a meta-analysis of the data, highlighting the need for high quality clinical trials to improve knowledge on long-term patient and disease outcomes. The use of purified phage to treat superficial bacterial infections appears to be efficacious safe, and side effect free, even when delivered by invasive routes of administration (e.g. intravenous and intra-articular ), or used in immunocompromised patients. Some would argue that phage therapy appears to have a better safety profile than antibiotics , and has a minimal impact on commensal flora , thus reducing the likelihood of opportunistic infections. Consistent with our natural exposure to phages, there are no reports of allergic responses to phages, potentially making them suitable alternatives for patients with antibiotic hypersensitivity. Because phages can kill bacterial cells quickly , release of endotoxins from lysed bacterial cells in severe infections is a potential safety concern. However, it has been demonstrated that phage lysis releases less endotoxin than beta-lactam antibiotics. Another potential issue with phage therapy is that phage cocktails might have effects on non-targeted bacteria and so affect the human microbiome. However, phage therapy does not appear to have an adverse effect on gut microbiota and has less of an effect on gut diversity than antibiotics, having a beneficial effect on gut health. Studies have suggested that bacteriophages can interact with eukaryotic cells, significantly influencing the functions of tissues, organs, and systems of mammals, including humans. In addition, there are concerns regarding the long-term impact of phages on the human immune system. In terms of effects on innate immunity , phages appear to induce anti-inflammatory responses. In terms of effects on the adaptive immune response , phages can be immunogenic, but are not very effective at inducing a specific immune response. With regards to phages inducing anaphylaxis , no cases have been reported. Autoimmune responses to phage therapy are a possibility, however, their immunomodulatory role, particularly in curbing inflammation means that phage therapy is being explored for the treatment of autoimmune liver diseases . Although the indications are that phage therapy is safe, with few adverse effects, current research on phage safety monitoring lacks sufficient and consistent data for regulatory purposes, which would require a standardized phage safety assessment to ensure a robust evaluation of the safety profile of phage therapy. Although the Australian STAMP protocol is not a randomised clinical trial, it does provide a framework for the collection of higher quality efficacy and safety data than individual case studies.