Beyond Vaccines: Tackling Severe Covid-19 Disease
SEVERE COVID-19 DISEASE
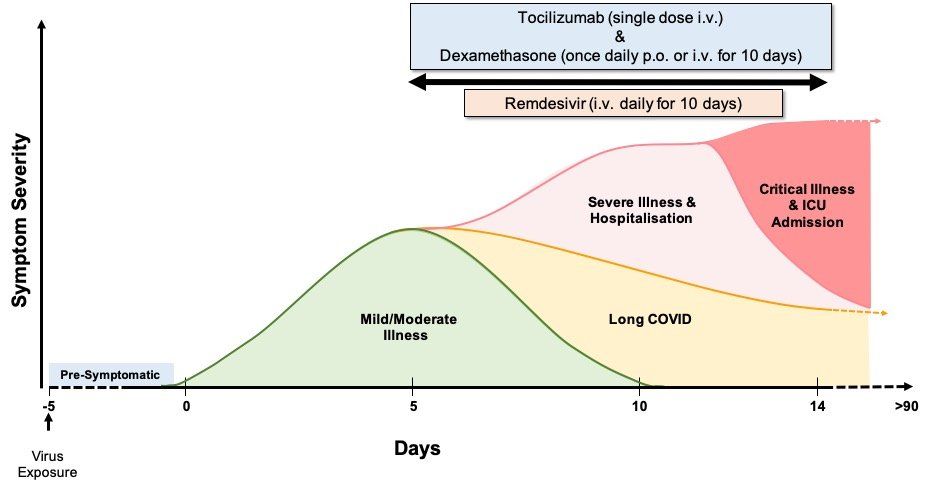
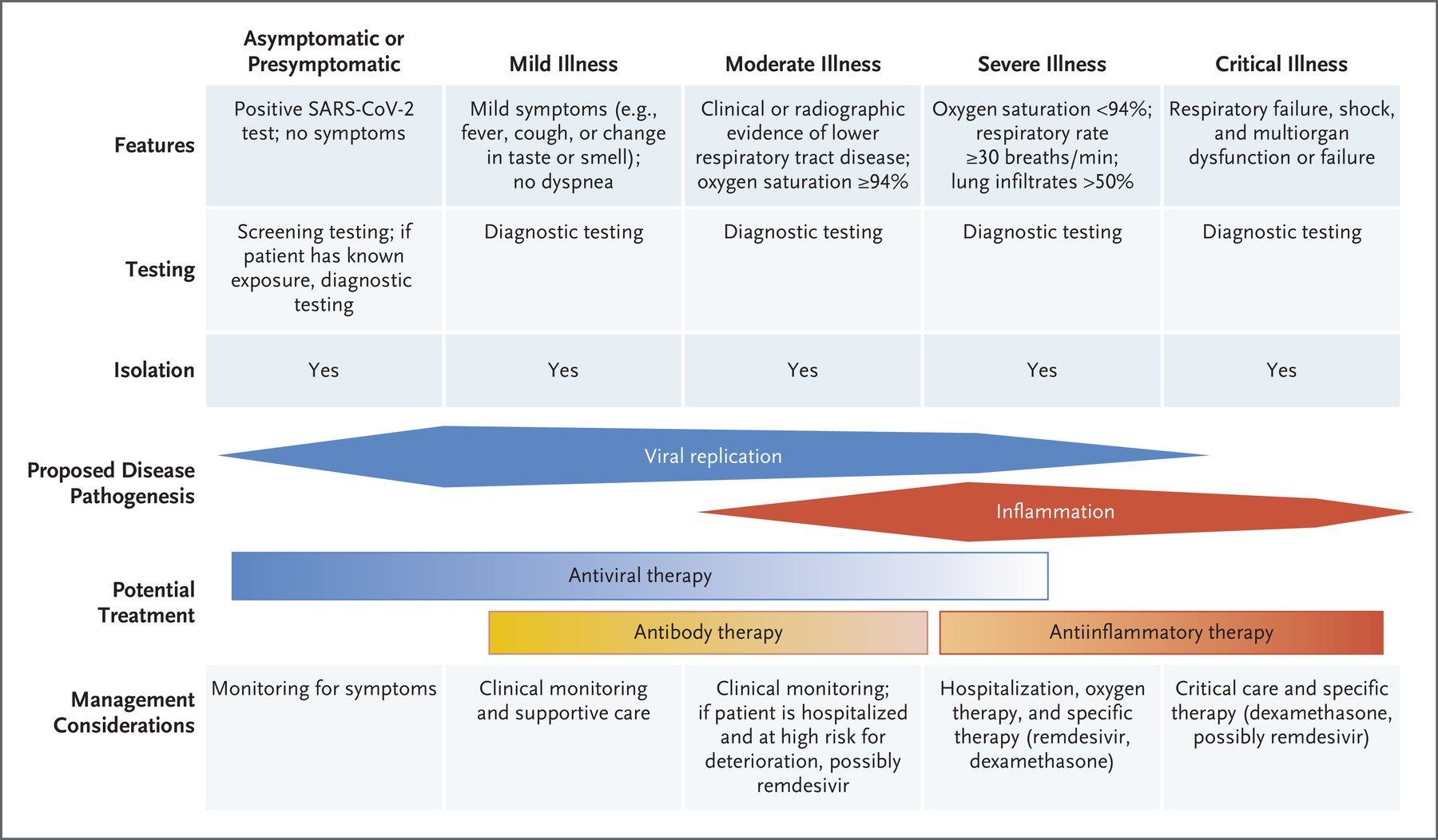
Around 20% of people infected with SARS-CoV-2 develop severe disease symptoms requiring hospitalisation and, in some cases, intensive care support for critical respiratory disease and/or multi-organ failure (Fig. 1.).
Fig. 1. COVID-19 symptomatology time course and approved drug treatments. COVID-19 symptoms become apparent around 5 days after initial virus exposure. Symptoms peak, on average, 5 days later and, for the majority (>60%) of people, COVID-19 symptoms are mild to moderate in severity. Although symptoms subside for 4 in 5 of these mild/moderate cases after another 5 days, 1 in 5 people can experience persistent symptoms for 12 weeks or more (long COVID). Twenty percent of infected individuals require hospitalisation with hypoxaemia caused by viral pneumonia. Of these hospitalised patients, 1 in 5 develop acute respiratory distress syndrome (ARDS) and/or multi-organ failure requiring intensive care. Up to 40% of critical care patients can die. Around 17% of infected people never develop any symptoms of COVID-19 at any time. So far, the only approved therapies are the anti-viral drug remdesivir and the immunomodulatory drugs dexamethasone and tocilizumab, used singly or in combination, for patients hospitalised with severe COVID-19. In randomised clinical trials remdesivir reduces time to recovery, whereas dexamethasone and tocilizumab reduce mortality in hypoxaemic patients receiving oxygen support, as well as reducing time to recovery and/or mortality, particularly when used in combination. Abbreviations: p.o. – by mouth; and i.v. -intravenous
The rollout of the AstraZeneca-Oxford and Pfizer-BioNtech SARS-CoV-2 vaccines is already having a major impact on the incidence of severe COVID-19 in the UK. Hopefully, similar effects will be seen as vaccines are rolled out in the EU and globally. However, despite the success in developing highly effective vaccines, there is still a need to develop other therapies. These include, antiviral drugs to directly target the SARS-CoV-2 virus, block virus entry/exit, or inhibit virus replication, as well as immunomodulatory drugs to prevent, or attenuate severe COVID-19. Developing these therapies is important given: vaccine hesitancy; the proportion of people in the world who have still to be vaccinated; and the continuing emergence of virus variants.
ANTI-VIRAL DRUGS
So far, remdesivir is the only anti-viral drug approved for treating Covid-19. Its use is limited to clinical settings (Fig. 1.), where it is reported to reduce time to recovery in hospitalised patients. A number of anti-viral drugs are currently being investigated for use in COVID-19. Interestingly, clinical trials of unapproved monoclonal antibody therapies in non-hospitalised adults with mild to moderate COVID-19 symptoms at high risk for progressing to severe COVID-19 have demonstrated reductions in hospitalisations and deaths. Potent, small molecule anti-viral drugs that could be given orally soon after symptom development, or after a positive RT-PCR test, to prevent hospitalisation in individuals at high risk of developing severe COVID-19, would be a valuable addition to the COVID-19 armamentarium.
IMMUNOMODULATORY DRUGS
Markers of inflammation are predictive of critical illness and mortality in COVID-19, and dysregulation of the host immune response is a key feature in individuals with severe disease, particularly those requiring critical care for COVID-19 ARDS. Analysis of data collected from COVID-19 patients already on immunomodulatory drugs for other diseases suggests that such drugs may provide protection from severe disease. In addition, a recent randomised controlled trial showed that early administration of the inhaled corticosteroid budesonide reduced the need for urgent medical care and reduced COVID-19 recovery time. Although children present with milder symptoms compared to adults, rare cases of a very severe multisystem inflammatory syndrome in children (MIS-C) have been reported. However, the hyper-inflammation observed in MIS-C differs from that seen in adults with severe COVID-19. Therefore, it may be that therapies for severe COVID-19 in adults and MIS-C may need to have different immunomodulatory profiles.
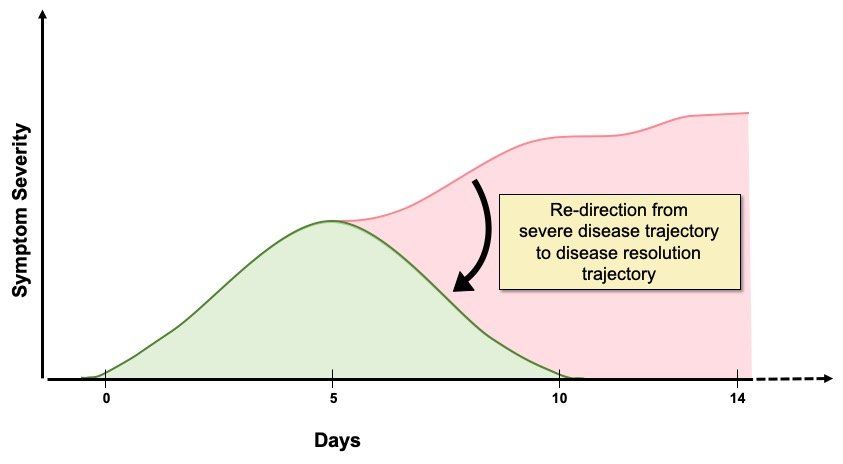
The value of immunomodulators in improving clinical outcomes in severe COVID-19 has been directly demonstrated by the RECOVERY trial. Administering the cheap and readily available corticosteroid dexamethasone to hospitalised severe COVID-19 patients with hypoxaemia (Fig. 1.) lowered mortality in those receiving invasive and non-invasive oxygen support, but not in non-hypoxaemic patients receiving no respiratory support. Although guidelines indicate reserving dexamethasone administration for patients requiring supplemental oxygen, a published study suggests that hypoxaemia should not be a trigger for dexamethasone usage unless the patient has been hospitalised for >72 hours. Key features in determining the timing of dexamethasone dosing are likely to be viral load and the level of inflammation in individual patients (Fig. 2.).
The RECOVERY trial also demonstrated that giving the more expensive humanized anti-human interleukin-6 (IL-6) receptor monoclonal antibody, tocilizumab, to patients hospitalised with severe COVID-19 (Fig. 1.) reduces the risk of death, shortens time until discharge, and reduces the need for mechanical ventilation. However, it is worth noting that there have been conflicting results from several randomised clinical trials of tocilizumab in COVID-19.
Importantly, the benefits of tocilizumab are seen on top of those provided by dexamethasone. Combination treatment reduces mortality for hypoxic patients requiring non-invasive and invasive mechanical ventilation. As a result, UK and US clinical guidelines now recommend the use of tocilizumab and dexamethasone in the treatment of hypoxaemic patients with severe COVID-19 requiring ventilation, but not for patients without hypoxaemia, or with significant immunosuppression. Although the RECOVERY trial did not explore optimal timing, dose, or duration, data from a Spanish study indicates that administering tocilizumab in combination with dexamethasone at hospital admission results in better outcomes than giving it 24 hours later.
The impressive results using tocilizumab and dexamethasone currently set the standard for severe COVID-19 immunomodulatory therapies, however, there is still scope for the development of other classes of immunomodulators with different mechanisms of action and different (or superior) side effect profiles, especially given the complexity of the innate and adaptive immune responses in severe COVID-19 and cytokine and cytokine receptor pleiotropy and redundancy.
A number of potential immunomodulatory therapies for severe COVID-19 have been suggested including, anti-cytokines, non-specific immune modulators, and specific signalling pathway modulators affecting pro-inflammatory cytokine production. Existing small molecule immunomodulatory drugs, or biologics targeting specific cytokines other than IL-6, developed for autoimmune or other inflammatory diseases, are being tested for being suitable for re-purposing as therapies for severe COVID-19. Alternative immunomodulators could lead to improved clinical outcomes (Fig. 2.), particularly in subsets of severe COVID-19 patients who do not respond to tocilizumab and dexamethasone therapy, or have specific co-morbidities, or different inflammatory profiles. Because systemic inflammation is important in driving pulmonary and extra-pulmonary damage in severe COVID-19, immunomodulatory drugs would be expected to be beneficial in addressing this aspect of the disease.
Could immunomodulators be useful in preventing or treating long COVID? Whether immunomodulators, potent anti-viral drugs, or a combination of the two, can have a positive impact on this post-COVID syndrome is unclear at the present time. This is because it is not known if the burden of health loss due to long COVID is a result of: long-term persistence of the virus; long term sequelae resulting from disruption of individuals’ immune and inflammatory responses; or due to something else entirely.
Fig. 2. Immunomodulatory drug treatment for severe COVID-19. The goal of immunomodulatory therapy in severe COVID-19 is to halt the patient’s journey along the severe disease trajectory and to re-direct them down the resolution trajectory instead. This reduces mortality, length of hospital stay, the need for ventilation and progression to critical illness. The degree and speed with which normal, or at least improved function, is restored will vary between different patients, depending on age and co-morbidities, but improvements will have a positive impact on clinical outcomes and healthcare resource usage and costs. In addition to the drug itself, dosage, timing, and duration of treatment are all likely to be important in terms of controlling severe COVID-19 disease progression, as well as minimising drug side effects. Administration of immunomodulatory drugs too early could negatively impact the host anti-viral response and actually encourage viral replication and spread in the respiratory tract, whereas effectively attenuating (not ablating) excess inflammation will be beneficial, as clinical studies using dexamethasone and tocilizumab have shown. The identification of blood biomarkers that distinguish between protective and damaging inflammatory mediator levels could be used to guide the timing of dosing. It is likely that early treatment of symptomatic/virus positive high risk individuals with a potent anti-viral drug to reduce viral load, followed by immunomodulatory therapy to attenuate hyperinflammation, if required, will be the most effective therapeutic strategy.
PROGNOSTIC BIOMARKERS
Early detection of severe COVID-19 will enable prioritization of high-risk individuals for treatment, positively impacting healthcare resource usage and costs. Factors including age, comorbidities, immune response, radiographic findings, laboratory markers, and indicators of organ dysfunction may individually, or collectively, predict worse outcomes. Although the U.S. Food and Drug Administration has issued an emergency use authorization for a machine learning-based device that may be used to indicate SARS-CoV-2 infection in certain asymptomatic individuals, no biomarker tests for severe COVID-19 have been authorised. However, blood proteins; cardiac injury markers; levels of COVID-19-neutralizing antibodies; markers of complement activation and endothelial damage; immune signatures; and cytokine levels, including GM-CSF, all have potential for use as biomarkers of COVID-19 severity. Given the range of clinical, molecular and biochemical readouts and bioinformatics tools available, my feeling is that the best biomarkers will be prognostic algorithms which predict COVID-19 severity by combining these different readouts to allow personalised immunomodulatory treatment strategies that reflect the underlying multifactorial pathophysiological mechanisms in different patients.
CONCLUSIONS
Despite the success of vaccination and social distancing measures in reducing COVID-19 incidence in the UK, as of 18th March 2021 there have been 127,508 COVID-19 related deaths. Globally there have been 3,009,215 deaths. These figures are still rising as the disease continues to have a major impact on human health and re-shapes social, economic and healthcare systems globally. The effects will continue to be felt for some time as SARS-CoV-2 likely becomes endemic.
Although vaccines will continue to be the primary tool in protecting the world’s population from SARS-CoV-2 infection and COVID-19 disease, there is still a need for the development of anti-viral and immunomodulatory drugs to contain the effects of the virus in individuals who develop severe disease symptoms and require hospitalisation. The results obtained with remdesivir, dexamethasone and tocilizumab in clinical trials demonstrate the value of these therapeutics in positively impacting clinical care and disease outcomes. However, there is a lot of scope for improvement, particularly for patients who do not respond to these therapies.
In addition to the drugs themselves, timing of drug dosing will be very important. Too early, and there is likely to be a negative effect on protective host immunity, encouraging virus replication and spread through the respiratory tract. Too late, and the ability to prevent or modify severe disease progression and limit long-term complications such as lung fibrosis and extra-pulmonary organ damage will be compromised. Blood biomarkers as well as clinical parameters will be an important tool for guiding clinical decisions on the timing and duration of drug dosing. Prognostic algorithms that combine clinical and physiological parameters, together with biochemical and immunological markers will be required, given the syndromic nature of COVID-19. For people at high risk of developing severe COVID-19, the use of data from wearable devices appears to be an effective way of monitoring high risk individuals to enable early anti-viral and immunomodulator treatment that may remove or reduce the need for hospitalisation and the need for critical care.
Clearly the benefits of reducing the need for hospitalisation, the length of hospital stay and the need for clinical intervention are enormous, particularly given the pressures that COVID-19 has placed on healthcare systems and the way it has impacted the treatment for non-COVID-19 disease. Finally, anti-viral and immunomodulatory drugs developed to treat COVID-19 could potentially be re-purposed in future pandemics resulting from different viruses.